Determination of individual blood compatibility. Tests for compatibility during blood transfusion
Details
When transfusing blood, the doctor must do the following:
1. Determine the indications for blood transfusion, identify contraindications, collect a transfusion history.
2. Determine the blood type and Rh factor of the recipient.
3. Select the appropriate (single-group and single-rhesus) blood and macroscopically evaluate its suitability.
4. Recheck the donor's blood type (from the vial) according to the ABO system.
5. Conduct a test for individual compatibility according to the ABO system.
6. Conduct a test for individual compatibility according to the Rh factor.
7. Conduct a biological test.
8. Perform blood transfusion.
9. Fill out the documentation.
COLLECTION OF TRANSFUSIOLOGICAL0G0 ANAMNESIS
It is necessary to find out from the patient whether he knows his group and Rh factor (used as additional information), whether there were transfusions of blood and its components in the past, whether there were any complications. In women, it is necessary to find out the presence of pregnancies and their complications (especially in Rh-negative women).
MACROSCOPIC EVALUATION OF BLOOD SUITABILITY
During visual inspection it is necessary to note:
■ Correctness.
■ Expiry date.
■ Tight packaging.
■ The blood should be divided into three layers (red erythrocytes at the bottom, a narrow gray band of leukocytes and platelets above, yellow transparent plasma above them).
■ Plasma must be clear, free of films and flakes (infected blood) and clots, and free of red color (hemolysis).
If at least one of the presented requirements is not met on macroscopic evaluation, such blood cannot be transfused.
TESTS FOR INDIVIDUAL COMPATIBILITY
Before setting up reactions, blood is taken from the recipient from a vein, which is divided into a clot and serum (by settling or centrifugation).
a) Test for individual compatibility according to the ABO system
A large drop (0.1 ml) of the recipient's blood serum and a small drop (0.01 ml) of the donor's blood from the vial are applied to a white surface and mixed with each other, periodically shaking the plate. The reaction is carried out at a temperature of 15-25°C, the results are evaluated after 5 minutes: the absence of agglutination of the donor's erythrocytes indicates the compatibility of the blood of the donor and the recipient according to the ABO system. The appearance of agglutination indicates their incompatibility - such blood cannot be transfused to this patient.
b) Test for individual compatibility by Rh factor
After the compatibility of the blood of the donor and the recipient according to the ABO system has been established, it is necessary to establish compatibility in relation to the Rh factor. The Rh factor compatibility test can be carried out in one of two ways:
■ test using 33% polyglucin,
■ assay using 10% gelatin.
In clinical practice, the most widely used test with polyglucin.
Sample using 33% polyglucin
The reaction is carried out in a centrifuge tube without heating for 5 minutes. 2 drops of the recipient's serum, 1 drop of donor blood and 1 drop of a 33% polyglucin solution are added to the bottom of the tube. After that, the contents are mixed by tilting the test tube and rotating it around its axis, distributing the contents over the walls in an even layer. The tube is rotated for 5 minutes, after which 3-4 ml of physiological saline is added and gently mixed, tilting the tube 2-3 times to a horizontal plane (without shaking!). After that, the result is evaluated: the presence of erythrocyte agglutination indicates the incompatibility of the blood of the donor and the recipient according to the Rh factor, such blood cannot be transfused. Uniform staining of the contents in the test tube, the absence of an agglutination reaction indicates the compatibility of the blood of the donor and recipient in terms of the Rh factor.
Sample using 10% gelatin
At the bottom of the tube, 1 drop of donor erythrocytes, previously washed with a tenfold volume of physiological saline, is placed, then 2 drops of a 10% gelatin solution heated to liquefaction and 2 drops of the recipient's serum are added.
Mix the contents of the tube and place in water bath at a temperature of 46-48 C for 10 minutes. After that, 6-8 ml of physiological saline is added to the tube, the contents are mixed, turning the tube 1-2 times and the result is evaluated: the presence of agglutination indicates the incompatibility of the blood of the donor and the recipient, its transfusion is unacceptable.
If the contents of the test tube remain uniformly colored and no agglutination reaction is observed in it, the donor's blood is compatible with the recipient's blood according to the Rh factor.
In some recipients (in the presence of incomplete latent or blocking antibodies, low activity of immune antibodies), these tests do not reveal incompatibility. In these cases, an individual selection of donor blood is carried out.
Individual selection of donor blood is necessary for the following groups of recipients:
1. Isoimmunized by previous blood transfusions or pregnancies.
2. Those who have undergone a blood transfusion complication.
3. In need of a massive blood transfusion.
4. If it is impossible to pick up compatible blood conventional compatibility tests.
BIOLOGICAL SAMPLE
Exists a large number of minor group systems that can cause complications. To exclude this possibility, at the beginning of a blood transfusion, another test for compatibility is performed - a biological test.
First, 10-15 ml of blood is transfused in a jet, after which the transfusion is stopped (the dropper is blocked) and the patient's condition is monitored for 3 minutes. With absence clinical manifestations reactions or complications (increased heart rate, respiration, shortness of breath, shortness of breath, flushing of the face, etc.) 10-15 ml of blood is reintroduced and the patient is observed again for 3 minutes. This is repeated three times.
The absence of reactions in the patient after a triple check is a sign of the compatibility of the infused blood and serves as the basis for the implementation of the entire blood transfusion.
If the blood of the donor and the recipient is incompatible during the biological test, the patient's behavior becomes restless: tachycardia, shortness of breath, flushing of the face, feeling of chills or heat, tightness in the chest, abdominal pain and very important feature- Pain in the lumbar region.
When these signs appear, the blood is considered incompatible and blood transfusion is not performed.
IMPLEMENTATION OF HEMO TRANSFUSION
In the absence of signs of biological incompatibility, drip blood transfusion is started. Prior to transfusion, the vial of transfused blood must be at room temperature within 30-40 minutes, and in emergency situations it is heated to 37 ° C in a water bath. Transfusion is carried out using a disposable blood transfusion system with a filter, usually at a rate of 40-60 drops per minute.
During blood transfusion, monitoring of the patient's condition continues. After transfusion, the container with the remnants of the transfusion medium (about 15 ml) and the recipient's serum are stored for 2 days in the refrigerator so that it is possible to analyze blood transfusion complications if they develop.
COMPLETING DOCUMENTATION
After the end of the transfusion, the doctor writes down the blood transfusion protocol in the medical history:
■ indications for transfusion,
■ passport data from each vial: donor's name, blood type, Rh-affiliation, vial number, date of blood collection,
■ blood type and Rh factor of the recipient and donor,
■ results of tests for individual blood compatibility of the donor and the recipient according to the ABO system and the Rh factor,
■ the result of a biological sample,
■ the presence of reactions and complications,
■ date, name of the doctor who transfused blood, signature.
OBSERVATION OF THE PATIENT AFTER HEMOTRANSFUSION
The recipient after blood transfusion observes for 2 hours bed rest and is observed by the attending and duty doctor during the day. Particularly careful monitoring is carried out during the first three hours after blood transfusion. The presence of complaints, changes in the general condition are assessed, body temperature, pulse rate and blood pressure are measured every hour. It is necessary to macroscopically evaluate the first portion of urine after blood transfusion, pay attention to the preservation of urination and urine color.
The next day, it is mandatory to perform a clinical blood test and general analysis urine.
TESTS FOR INDIVIDUAL COMPATIBILITY OF BLOOD DONOR AND RECIPIENT
The purpose of the individual compatibility test is to prevent transfusions of incompatible red blood cells. Testing the recipient's serum with the RBCs of the intended donor is the most reliable way detection of antibodies that can cause damage to transfused erythrocytes, post-transfusion reactions, including hemolytic ones. This test allows you to:
- confirm ABO compatibility of the donor and recipient;
- identify all antibodies in the recipient's serum directed against the donor's erythrocyte antigens.
Test for individual blood compatibility for antigens of the ABO system
Definition progress:
- on a clean, dry surface of a tablet or plate at room temperature, apply and mix the recipient's serum and donor's blood in a ratio of 10: 1;
- periodically shaking the tablet, observe the course of the reaction;
- in the absence of agglutination within 5 minutes, the blood is compatible. The presence of agglutination indicates the incompatibility of the blood of the recipient and the donor. This blood cannot be transfused. In doubtful cases, the result of the test is monitored under a microscope: in the presence of "coin columns" that disappear after adding a warm (+37 ° C) 0.9% sodium chloride solution, the blood is compatible; if agglutinates are visible in a drop of the mixture, which do not disperse when a warm 0.9% sodium chloride solution is added, the blood is incompatible.
Blood compatibility test for antigens of the Rhesus system using a 33% solution of polyglucin
Since the sensitivity of this test is low, use it in stationary medical institutions Not recommended. It is allowed to perform a compatibility test using a 33% solution of polyglucin if it is necessary to conduct a blood transfusion in extreme conditions.
The compatibility test for antigens of the Rhesus system does not replace, but complements the compatibility test for antigens of the ABO system.
Definition progress:
- 2 drops of the patient's serum, one drop of donor blood and one drop of a 33% polyglucin solution are added to the bottom of the labeled tube;
- mix the contents of the tube by shaking;
- slowly turn the tube so that the contents spread over the walls of the tube. This increases the severity of the agglutination reaction;
- after 5 minutes add 2-3 ml of saline;
- mix the contents by gently inverting the tube 2-3 times without shaking;
- the presence of agglutination in the tube indicates that the donor's blood is incompatible with the patient's blood and therefore cannot be transfused. If the contents of the tube remain uniformly colored and there are no signs of erythrocyte agglutination, the blood is compatible.
Test for individual blood compatibility using centrifugation
Definition progress:
- place 2 drops of the recipient's serum at the bottom of the labeled tube;
- add 1 drop of a 5% suspension of three times washed donor erythrocytes in a solution of low ionic strength - RNIS (in the absence of RNIS, saline can be used, in which case the fixation of antibodies worsens). The method for preparing a 5% suspension of washed erythrocytes three times is given in the description of the indirect Coombs test;
- immediately after mixing the donor's erythrocytes and the recipient's serum, the sample is centrifuged for 15-20 s at 2000 rpm;
- gently shaking the tube, resuspend the cell sediment and visually determine the presence of agglutinates. The presence of hemolysis and/or agglutinates may mean:
- a) incompatibility according to the ABO system;
- b) the presence in the serum of the recipient of complete cold antibodies of a different specificity (anti-M, anti-N, anti-S, etc.).
Test for individual blood compatibility using gelatin solution
It is necessary to use pure, without flakes, transparent gelatin, hardening at a temperature of +4°C. Gelatin should not be frozen. To exclude non-specific agglutination of erythrocytes with a gelatin solution, it is advisable to check each series of gelatin in the control with non-sensitized erythrocytes.
Definition progress:
- heated 10% gelatin solution in a water bath to liquefy (+46-48°C) for 10 minutes;
- 2 drops of a heated 10% gelatin solution are added to a mixture of donor erythrocytes and recipient serum (in a ratio of 1: 2) and mixed thoroughly;
- for 10 min incubate the test tube at +46-48°C in a water bath or for 30 min in a thermostat;
- add 5-8 ml of saline and mix gently, inverting the tube 1-2 times;
- visually determine the presence or absence of agglutination of erythrocytes. At negative result transfer a drop of a suspension of erythrocytes from a test tube to a glass slide and microscope at low magnification.
The following samples are used as a negative control:
- a) a mixture of donor erythrocytes with gelatin and 0.9% sodium chloride solution (in a ratio of 1:2:2);
- b) a mixture of erythrocytes and serum of the recipient with gelatin (in the ratio 1:2:2).
Test for individual blood compatibility using an antiglobulin reagent
Do not re-freeze-thaw antiglobulin reagent. To control the quality of the antiglobulin reagent, it is advisable to conduct an antiglobulin test with Rh-positive erythrocytes sensitized with IgG (incomplete) anti-D antibodies.
Definition progress:
Conduct a test for individual blood compatibility using centrifugation. If no hemolysis is detected, and the erythrocytes form a homogeneous suspension after shaking the tube:
- incubate the tube at 37°C for: 15 min when using RNIS, or 45 min when using saline;
- centrifuge the tube for 15-20 s at 2000-3000 rpm;
- visually determine the presence of hemolysis in the supernatant;
- gently shaking the tube, resuspend the cell sediment and visually determine the presence of agglutinates. The presence of hemolysis and/or agglutinates indicates that the recipient has complete warm antibodies to the donor's erythrocyte antigens;
- in the absence of hemolysis and agglutination, the erythrocytes are thoroughly washed 3-4 times, each time using 5-10 ml of saline;
- remove saline;
- add 1-2 drops of antiglobulin reagent to the erythrocyte sediment and mix thoroughly;
- centrifuge for 15-20 s at 2000-3000 rpm;
- gently resuspend the erythrocyte sediment and visually determine the presence or absence of agglutination;
- visually determine the presence or absence of agglutination of erythrocytes.
If the result is negative, a drop of erythrocyte suspension is transferred from the test tube to a glass slide and microscoped at low magnification.
When agglutination is detected, the blood of the donor and the recipient is incompatible for erythrocyte antigens.
As negative control samples use:
- a mixture of 5% suspension of test erythrocytes with 0.9% sodium chloride solution (in a ratio of 1:2) - control for spontaneous agglutination;
- a mixture of 5% suspension of test erythrocytes with an anti-globulin reagent (in a ratio of 1: 2) - control of autosensitization.
The control of the activity of the antiglobulin reagent (always a positive reaction) is a mixture of 5% suspension of donor erythrocytes of group 0 (I) D + sensitized with anti-Rh serum (containing anti-D antibodies of the IgG class) and an antiglobulin reagent (in a ratio of 1:2).
Monitoring the specificity of the antiglobulin reagent (always backlash) - a mixture of 5% suspension of donor erythrocytes of group 0 (I) D - (after incubation with anti-Rhesus serum for 45 minutes) and an antiglobulin reagent (in a ratio of 1: 2).
Store the antiglobulin reagent frozen at -20°C (dry reagent can be stored at room temperature). Repeated freezing-thawing of the reagent is unacceptable.
Reasons for false positive results:
- the presence of autoantibodies on the surface of test erythrocytes;
- test erythrocytes contain microbial impurities;
- violation of the centrifugation mode ( mechanical damage erythrocyte membranes under excessive load).
Reasons for false negative results:
- test erythrocytes are poorly washed, or the remains of serum proteins are present on the walls of the test tube;
- violation of the regimes of laundering or incubation;
- loss of activity of serum or test erythrocytes during storage;
- low activity of the antiglobulin reagent;
- insufficient incubation time.
| Page
Source: Medical laboratory diagnostics, programs and algorithms. Ed. prof. Karpishchenko A.I., St. Petersburg, Intermedica, 2001 |
When transfusing erythrocyte-containing blood components, in addition to determining the blood type and Rh factor, two tests are performed to identify the presence of complete and incomplete antibodies to the donor's erythrocytes in the recipient. Besides, in without fail a three-fold biological test is carried out.
When transfusing plasma and its preparations, only a three-fold biological test is performed.
When transfusing blood components from several vials or containers to a patient, compatibility tests should be made with the contents of each vial or container, even if they indicate that the blood was received from the same donor.
Total antibody test
Previously called "Test for individual compatibility", "Cold test". Allows you to identify agglutinins to antigens of the ABO, MNSs, Lewis, etc. systems.
The test is performed with the recipient's blood serum, which is obtained by centrifugation or settling of blood. Serum is suitable for use provided it is stored in a refrigerator at a temperature of +4ºС for no more than 2 days.
2-3 drops of the patient's blood serum are applied to a white plate, to which a 5-fold smaller drop of blood or erythrocyte mass of the donor (from the container) is added - the ratio of blood and serum is approximately 1:10. The blood is mixed with serum with a glass rod with a melted end, then the plate is shaken for 5 minutes, observing the course of the reaction. After the specified time, it is recommended to add 1-2 drops of physiological saline to the reacting mixture to eliminate "false agglutination".
The absence of agglutination indicates the compatibility of the blood of the donor and the recipient, and the appearance of agglutination indicates their incompatibility and the inadmissibility of this blood transfusion.
Incomplete antibody test
Previously called "Rh factor compatibility test", "Heat test".
It can be done in several ways. The most sensitive is the indirect Coombs test. However, its implementation is quite difficult, it requires a special reagent (anti-Rhesus serum) and considerable time (about 2 hours). As a result, the Coombs test is performed only under laboratory conditions for special indications, for individual selection blood to "difficult" recipients. It is somewhat inferior to it in accuracy, but a test with a 10% gelatin solution in a water bath is technically easier. A test with a 33% polyglucin solution is technically the simplest and fastest, but also the least accurate. For the needs of practical public health, the most suitable is a sample with a 10% gelatin solution in a water bath.
Rh factor compatibility test using 10% gelatin solution.
The essence of the reaction is to create a colloidal medium and elevated temperature, in which incomplete antibodies are adsorbed on gelatin molecules and, interacting with the surface antigens of erythrocytes, agglutinate them.
The sample is made in test tubes at a temperature of + 46-48ºС for 10 - 15 minutes. At the bottom of the test tube, respectively designated, place 1 drop of the donor's erythrocytes, then add 2 drops of the patient's serum and 2 drops of a 10% gelatin solution heated to liquefaction. The gelatin solution must be carefully examined before use. When turbidity or the appearance of flakes, gelatin is unsuitable. The contents of the tube are mixed by shaking and placed in a water bath at a temperature of + 46-48ºС for 10 - 15 minutes or in a thermostat at the same temperature for 30 minutes. Then, 5-8 ml of isotonic sodium chloride solution is added to the test tube, the contents are mixed by 1-2 repeated inversion of the test tube and viewed in the light with the naked eye or through a magnifying glass.
The presence of agglutination in the form of a suspension of small, less often - large lumps against the background of a clarified or completely discolored liquid means that the donor's blood is incompatible with the patient's blood and cannot be transfused to him.
If the contents of the tube remain uniformly colored, slightly opalescent, there is no agglutination of erythrocytes, then the donor's blood is compatible with the patient's blood in relation to the Rh factor Rh (D).
NB ! In the Instructions of 2002, in comparison with the previous instructional documents, changes were made to the technique of setting a sample with 10% gelatin in a water bath. Previously, the residence time in the water bath was 10 min. new instructions- 15 minutes. Introduced the use of a thermostat instead of a water bath.
Rh factor compatibility test using 33% polyglucin solution.
The essence of the test is the same as the previous one, but the temperature factor is excluded.
The sample is carried out in a test tube without heating for 5 minutes. 2 drops of the patient's serum, 1 drop of donor blood and 1 drop of a 33% solution of polyglucin, specially produced for this purpose in 5 ml vials, are added to the bottom of the test tube, previously designated. The contents of the tube are mixed by shaking, then the tube is tilted to an almost horizontal level and slowly rotated so that the contents of the tube spread over the walls. This procedure is continued for 5 minutes. Then, 3-4 ml of isotonic sodium chloride solution is added to the test tube, mixed by 2-3 times smooth inversion of the tube, closing it with a stopper and looking at the light with the naked eye.
If there are agglutinates in the solution against the background of a clarified or completely discolored liquid, a conclusion is given that the donor's blood is incompatible with the patient's blood and cannot be transfused to him. If the contents of the test tube remain evenly colored, without signs of agglutination, then a conclusion is made about the compatibility of the blood of the patient and the donor in terms of Rh o (D) factor.
Compatibility test before transfusion of blood components. Execution technology. Manual for doctors./GOU DPO KSMA Roszdrav. - Kazan, 2011. - 35 p.
The manual presents technologies for performing tests for individual compatibility before transfusion of donor blood components in order to prevent post-transfusion complications of the hemolytic type. The requirements for blood samples, for specialists conducting tests, as well as the procedure for conducting tests, depending on the type of transfused transfusion medium and the characteristics of conducting tests in certain categories patients.
The manual contains a list of equipment and reagents required for testing for individual compatibility.
It is intended for doctors of all specialties conducting blood transfusion therapy. It can be used in the training of specialists in higher and secondary medical educational institutions.
FROM study guide can be found in the library of GOU DPO KSMA Roszdrav at 420012, Kazan, st. Mushtari, 11
Introduction. The purpose of testing for individual compatibility is to prevent transfusions of incompatible blood components. Compatibility refers to a favorable combination of donor and recipient blood. Their biologically impossible combination of antigens and antibodies of different group systems determines the incompatibility of the blood of the donor and the recipient.
The ABO blood group is a set of group antigens A and B and natural anti-A, anti-B antibodies, which is hereditarily determined and does not change throughout life.
The Rhesus system is one of the most polymorphic antigenic systems of human erythrocytes. It includes about 50 serologically different antigens, not counting the weak, transitional and partial forms. On human erythrocytes there are 5 main antigens of the Rhesus system (D, C, c, E, e). Greatest clinical significance has antigen D. Possessing pronounced immunogenic properties, antigen D in 95% is the cause of hemolytic disease of the newborn, as well as the cause of severe post-transfusion complications. Persons with the D antigen are referred to as Rh positive (Rh+), those who do not have the D antigen are referred to as Rh negative (Rh-).
Currently, more than 236 erythrocyte antigens are known, which are distributed in 29 genetically independent systems. Antigens of the ABO and Rhesus systems are of paramount clinical importance. Erythrocyte systems Kell, MNS, Levis, Duffi, Kidd have less practical value, as they relatively rarely cause sensitization. These systems become significant in multiple blood transfusions of red blood cells, repeated pregnancies. Antibodies to all these antigens can be formed in a person of any blood group of the ABO system, Rhesus (regardless of Rh affiliation). They are formed under the same conditions as anti-D antibodies (multiple blood transfusions of red blood cells, repeated pregnancies) and can cause post-transfusion complications of the hemolytic type and hemolytic disease of the newborn. If a recipient whose blood contains antibodies is transfused with the blood of a donor whose erythrocytes contain antigens against which antibodies are directed, such blood will be destroyed in the recipient's body, i.e. she is incompatible with him.
This manual presents the technology for performing tests for individual compatibility, provides requirements for blood samples, for specialists conducting tests, as well as the procedure for conducting tests depending on the type of transfused transfusion medium and the characteristics of conducting tests in certain categories of patients.
Technology for performing compatibility tests before transfusion of blood components:
1. Characteristics of the methodology for performing tests for compatibility before transfusion of blood components
1.1. Requirements for blood samples
Before the procedure for taking blood for testing for compatibility, the recipient's last name, first name and patronymic are specified. They take a dry, clean test tube, on which the following information about the patient is applied: full name, date of blood sampling, for inpatients - the number of the medical card. The patient's blood is taken from a vein in an amount of 3-5 ml and transferred to a signed tube. To obtain serum, the tube with the blood sample is left at room temperature for at least 1 hour or, in emergency cases, centrifuged in a laboratory centrifuge at a speed of 1500-2000 rpm. within 5 minutes. The recipient's blood sample is used, stored at t+2 0 С…+8 0 С for no more than two days.
Hemolyzed blood is not used for research. In the presence of hemolysis, the blood sampling procedure is repeated.
The pre-transfusion recipient's blood sample is stored at +2 0 С…+8 0 С for 5 days. This is necessary for further examination of the recipient's blood in a specialized blood service laboratory in the event of a post-transfusion complication.
In the event of a post-transfusion complication, the recipient's pre-transfusion blood sample and the remains of the transfusion medium are sent to a specialized blood service laboratory for analysis.
1.2. Requirements for the premises
Immunohematological studies are carried out in a room with natural light, the air temperature in the room should be within +15 0 С ... +25 0 С.
1.3. General requirements for testing for compatibility
Before transfusion of blood components, tests are carried out for individual compatibility according to the ABO and Rhesus systems, and a biological sample.
When testing for individual compatibility according to the ABO system, compatibility according to the MNS system is also detected, and when testing for individual compatibility according to the Rhesus system, compatibility is also detected according to other clinically significant blood group systems (Kell, MNS, Levis, Duffi, Kidd).
Compatibility tests immediately before each transfusion are carried out by the doctor who transfuses the canned blood and its components, after the control redefinition of the blood group of the recipient and the donor, and the control redefinition of the Rh - belonging of the recipient's blood. The urgency of transfusion does not exempt from testing for compatibility.
1.4. Requirements for the scope of studies, depending on the type of transfused transfusion medium
1.4.1. Transfusion of blood gas carriers
Before transfusion of blood gas carriers, tests are carried out for individual compatibility of the blood of the recipient and the donor, including the auto-donor, according to the ABO and Rhesus systems, as well as a biological test after conducting control studies of the group and Rh belonging of the patient's blood and the group affiliation of the erythrocytes of the donor (auto-donor).
Compatibility tests for ABO and Rhesus blood types are carried out separately, they cannot replace each other, since antibodies different nature require different methods for your discovery.
A test for individual compatibility according to the ABO system is carried out according to one of the following methods: a test for compatibility on a plane at room temperature or in a gel test.
A test for individual compatibility according to the Rhesus system is carried out according to one of the following methods: a test using a 33% polyglucin solution, a test using a 10% gelatin solution or in a gel test.
1.4.2. Transfusion of correctors of hemostasis and fibrinolysis, means of correcting immunity
Before transfusion of hemostasis and fibrinolysis correctors, means of correcting blood immunity, a biological test is performed.
1.5. Requirements for specialists
Testing for individual compatibility is carried out by specialists trained in immunohematology at specialized advanced training courses based on institutions of additional vocational education and (or) on the basis of blood service institutions of the Republic of Tatarstan (see Table 1).
Table 1
Requirements for professionals to perform compatibility tests before transfusion of blood components
|
Medical institution |
Performing compatibility tests before transfusion of blood components |
medical staff |
Additional requirements for medical personnel |
|
Hospital, including a day hospital, in an outpatient setting |
Before each transfusion of blood and its components |
Physician directly administering the transfusion |
Must have special training in immunohematology at specialized advanced training courses on the basis of institutions of additional professional education and (or) on the basis of institutions of the blood service of the Republic of Tatarstan at least once every 5 years |
2. Material resources
To perform compatibility tests before transfusion of blood components, a separate place (or a special room) must be equipped, which is equipped in accordance with the requirements of the order of the Ministry of Health of the Russian Federation No. 2 of 01/09/1998 "On approval of instructions for immunoserology".
2.1 Devices, tools, products medical purpose
table 2
Devices, instruments, medical products necessary for performing compatibility tests, as well as alternative devices and medical equipment products.
|
Name of device, product |
Number of devices |
Alternative device, product of medical equipment |
|
Compatibility test for blood groups of the ABO system (on the plane) |
||
|
Table laboratory |
Plastic coated table |
|
|
Desk lamp |
Wall lamp |
|
|
Plates for immunohematological studies |
1 PC. for 1 study |
Plate white color porcelain or enameled |
|
Pasteur pipette |
||
|
glass rods (shoulder blades) |
From 1 to 10 pcs. |
plastic stick |
|
Hourglass for 5 minutes |
Stopwatch |
|
|
glass glasses |
glass jars |
|
|
Laboratory centrifuge OPN-3 |
Centrifuge laboratory | |
|
using 33% polyglucin solution (in vitro) |
||
|
Table laboratory |
Plastic coated table |
|
|
Desk lamp |
Wall lamp |
|
|
Centrifuge tube |
biological test tube |
|
|
Pasteur pipette |
Variable volume automatic pipette (20-200 µl) |
|
|
Hourglass for 5 minutes |
Stopwatch |
|
|
Loupe with 4-6x magnification |
||
|
10-socket laboratory stand |
||
|
glass glasses |
glass jars |
|
|
Laboratory centrifuge OPN-3 |
Centrifuge laboratory | |
|
Rhesus compatibility test using 10% gelatin solution (in vitro) |
||
|
Table laboratory |
Plastic coated table |
|
|
Desk lamp |
Wall lamp |
|
|
Water bath at 46-48 0 С |
Thermostat for 46-48 0 С |
|
|
Pasteur pipette |
Variable volume automatic pipette (20-200 µl) |
|
|
Thin-walled test tubes (capacity not less than 10 ml) |
Centrifuge tube |
|
|
Hourglass for 15 minutes |
Stopwatch |
|
|
Loupe with 4-6x magnification |
||
|
10-socket laboratory stand |
||
|
glass glasses |
glass jars |
|
|
Laboratory centrifuge OPN-3 |
Centrifuge laboratory | |
|
Table laboratory |
Plastic coated table |
|
|
Desk lamp |
Wall lamp |
|
|
Variable volume automatic pipette |
5-40 µl 1 pc. 100-1000 µl 1 pc. |
|
|
10-socket laboratory stand |
||
|
glass glasses |
glass jars |
|
|
Laboratory centrifuge OPN-3 |
Centrifuge laboratory | |
|
Gel card centrifuge |
||
|
Incubator at 37 0 C |
Thermostat at 37 0 С |
|
|
Centrifuge tube |
biological test tube |
|
2.2. Reagents
To perform tests for individual compatibility before transfusion of blood and its components, reagents are required, which are presented in Table 3.
Table 3
Reagents required for compatibility testing
|
Reagent name |
Document regulating the use |
Amount of Reagent Required to Run |
Terms and features of storage of the reagent, work with it* |
|
|
ABO compatibility test flat at room temperature |
||||
|
Sodium chloride solution, 0.9% |
Stored at t+15…+25 0 С. |
|||
|
Compatibility test using 33% polyglucin solution |
||||
|
Polyglucin solution, 33.0% |
Order of the Ministry of Health of the Russian Federation of November 25, 2002 No. 363 "On approval of the Instructions for the use of blood components" |
Stored at |
||
|
Sodium chloride solution, 0.9% |
Stored at |
|||
|
Compatibility test using 10% gelatin solution |
||||
|
Gelatin solution, 10.0% |
Order of the Ministry of Health of the Russian Federation of November 25, 2002 No. 363 "On approval of instructions for the use of blood components" |
Stored at |
||
|
Sodium chloride solution, 0.9% |
Stored at |
|||
|
Compatibility test according to the ABO system and the Rhesus system using gel diagnostic cards |
||||
|
Coombs+ diagnostic cards |
Instructions included with the diagnostic cards |
From 1/4 to 1/3 pcs. depending on the used diagnostic card. |
Stored at t+15…+25 0 С. |
|
|
Solution for preparing a suspension of test erythrocytes |
Instructions attached to the solution |
From 0.5 to 1.0 ml, depending on the manufacturer |
Stored at |
|
* - The temperature inside the cold room, where the reagents are stored, is controlled by the paramedic twice a day. Thermometer readings are recorded in the "Registration Log temperature regime storage of diagnostic standards for immunohematological studies” (see Annex 1).
2.3 Other consumables
Other consumables are used to perform compatibility tests prior to transfusion of blood components (see Table 4).
Table 4
|
Name |
Quantity |
Note |
|
Cotton gauze swab |
For 1 tampon: cotton wool - 3.0 gr., bandage - 30 cm. |
For drying pipettes and glass rods |
|
Disposable medical gloves |
1 pair for every 3 hours of work |
Replace immediately if broken or heavily soiled. |
|
Sodium chloride solution, 0.9% |
For washing pipettes and sticks |
|
|
Ethyl alcohol, medical, 70.0% |
3.0 gr. for 1 treatment |
For processing the hands of the staff after finishing work |
|
Disinfectant solution |
In accordance with the methodological guidelines for the use of misinformation. funds permitted for use in the Russian Federation and the Republic of Tatarstan |
For disinfection biological material, laboratory glassware and workplace. |
|
Tips for variable volume automatic pipettes, disposable |
4 things. for 1 study |
3. The procedure for testing for compatibility
The purpose of testing for compatibility is to prevent the transfusion of banked blood and its components that are incompatible with the blood of the recipient. For compatibility tests, the recipient's blood serum and a donor's blood sample are used.
If the patient is transfused with canned blood and its components from several containers, compatibility tests are carried out with each container, even if they indicate that the blood components were obtained from the same donor.
3.1 Tests for individual compatibility according to the ABO system
3.1.1 Compatibility test for ABO system blood types (on the plane)
The test is carried out on a wetted surface plate.
1. The tablet is marked, for which the full name is indicated. and blood group of the recipient, full name and the donor's blood group and blood container number.
2. Serum is carefully taken from the test tube with the recipient's blood to be tested and applied to the tablet 1 with a large drop (100 µl).
3. A small drop (10 µl) of donor erythrocytes is taken from a tube segment of a plastic bag with transfusion medium, which is prepared for transfusion to this particular patient, and applied next to the recipient's serum (serum to erythrocyte ratio 10:1).
4. Drops are mixed with a glass rod.
5. Observe the reaction for 5 minutes, while constantly shaking the tablet. After this time, 1-2 drops (50-100 µl) of sodium chloride solution, 0.9% are added.
the reaction in the drop can be positive or negative.
a) a positive result (+) is expressed in agglutination of erythrocytes, agglutinates are visible to the naked eye in the form of small or large red aggregates. The blood is incompatible, it is impossible to transfuse! (see figure 1).
Figure 1. Donor and recipient blood is incompatible
b) with a negative result (-), the drop remains homogeneously colored red, agglutinates are not detected in it. The donor's blood is compatible with the recipient's (see Figure 2).
Figure 2. Donor blood is compatible with recipient blood
 3.2. Tests for individual compatibility according to the Rhesus system
3.2. Tests for individual compatibility according to the Rhesus system
3.2.1. Compatibility test using 33% polyglucin solution
The order of the study:
1. For research, take a test tube (centrifuge or any other, with a capacity of at least 10 ml). The tube is labeled, for which the full name is indicated. and blood group of the recipient, and full name of the donor, the number of the container with blood.
2. Serum is carefully taken from the tube with the recipient's blood to be tested with a pipette and 2 drops (100 µl) are added to the bottom of the tube.
3. One drop (50 µl) of donor erythrocytes is taken from a segment of the tube of a plastic bag with a transfusion medium, which is prepared for transfusion to this particular patient, into the same tube, 1 drop (50 µl) of a 33% polyglucin solution is added.
4. The contents of the test tube are mixed by shaking and then slowly turned along the axis, tilting almost to a horizontal position so that the contents spread over its walls. This procedure is performed within five minutes.
5. After five minutes, add 3-5 ml of saline to the test tube. solution. The contents of the test tubes are mixed by inverting the test tubes 2-3 times (without shaking!)
Interpretation of reaction results:
the result is taken into account by looking at the test tubes in the light naked eye or through a magnifying glass.
If agglutination is observed in the test tube in the form of a suspension of small or large red lumps against the background of a clarified or completely discolored liquid, then the donor's blood is not compatible with the recipient's blood. You can't overflow!
If there is a uniformly colored, slightly opalescent liquid in the test tube without signs of erythrocyte agglutination, this means that the donor's blood is compatible with the recipient's blood in relation to antigens of the Rhesus system and other clinically significant systems (see Figure 3).
Figure 3. The results of the study of samples for compatibility according to the Rhesus system (using a 33% polyglucin solution and a 10% gelatin solution)
 3.2.2. Compatibility test using 10% gelatin solution
3.2.2. Compatibility test using 10% gelatin solution
The gelatin solution must be carefully examined before use. When turbidity or the appearance of flakes, as well as the loss of gelatinous properties at t + 4 0 С ... + 8 0 С, gelatin is unsuitable.
The order of the study:
1. Take a test tube for research (capacity not less than 10 ml). The test tube is marked, for which the full name, blood group of the recipient and donor, and the number of the container with blood are indicated.
2. One drop (50 µl) of donor erythrocytes is taken from a segment of the tube of a plastic bag with a transfusion medium, which is prepared for transfusion to this particular patient, put into a test tube, 2 drops (100 µl) of a 10% gelatin solution heated in a water bath are added to liquefaction at a temperature of +46 0 C ... +48 0 C. Carefully take the serum from the tube with the recipient's blood with a pipette and add 2 drops (100 μl) to the bottom of the tube.
3. The contents of the tube are shaken to mix and placed in a water bath (t+46 0 С…+48 0 С) for 15 minutes or in a thermostat (t+46 0 С…+48 0 С) for 45 minutes.
4. After the end of the incubation, the tube is removed, 5-8 ml of saline is added. solution, the contents of the tube are mixed by one or two inversions and the result of the study is evaluated.
Interpretation of the results of the reaction.
the result is taken into account by viewing the tubes in the light with the naked eye or through a magnifying glass, and then viewed by microscopy. To do this, a drop of the contents of the test tube is placed on a glass slide and viewed under low magnification.
If agglutination is observed in the test tube in the form of a suspension of small or large red lumps against the background of a clarified or completely discolored liquid, this means that the donor's blood is incompatible with the recipient's blood and should not be transfused to him.
If the test tube contains a uniformly colored, slightly opalescent liquid without signs of erythrocyte agglutination, this means that the donor's blood is compatible with the recipient's blood in relation to antigens of the Rhesus system and other clinically significant systems (see Figure 3).
3.3. Gel Compatibility Test
When setting up in a gel test, compatibility tests are carried out immediately according to the ABO system (in the Neutral microtube) and a compatibility test according to the Rhesus system (in the Coombs microtube).
The order of the study:
1. Before the study, check the diagnostic cards. Do not use cards if there are suspended bubbles in the gel, the microtube does not contain a supernatant, a decrease in the volume of the gel or its cracking is observed.
2. Microtubes are signed (name of the recipient and number of the donor sample).
3. From a segment of the tube of a plastic bag with a transfusion medium, which is prepared for transfusion to this particular patient, 10 μl of donor erythrocytes are taken with an automatic pipette and placed in a centrifuge tube.
4. Add 1 ml dilution solution.
5. Open required amount microtubes (one each of Coombs and Neutral microtubes).
6. Using an automatic pipette, add 50 µl of diluted donor erythrocytes to Coombs and Neutral microtubes.
7. Add 25 µl of recipient serum to both microtubes.
8. Incubate at t+37 0 C for 15 minutes.
9. After incubation, the card is centrifuged in a gel card centrifuge (time and speed are set automatically).
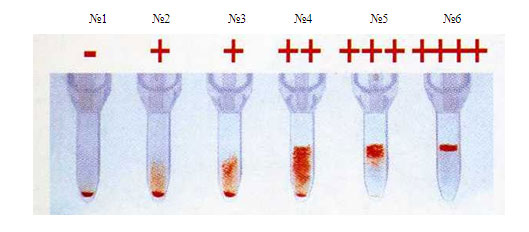
Interpretation of results:
if the erythrocyte sediment is located at the bottom of the microtube, then the sample is considered compatible (see Figure 4 No. 1). If agglutinates linger on the surface of the gel or in its thickness, then the sample is incompatible (see Figure 4 Nos. 2-6).
№1 №2 №3 №4 №5 №6
Figure 4. The results of the study of samples for individual compatibility according to the Rhesus system by the gel method
 3.4. biological sample
3.4. biological sample
To conduct a biological test, blood and its components prepared for transfusion are used.
A biological sample is carried out regardless of the volume of the blood transfusion medium and the rate of its administration. If it is necessary to transfuse several doses of blood and its components, a biological test is carried out before the start of transfusion of each new dose.
Technique:
10 ml of blood transfusion medium is transfused once at a rate of 2-3 ml (40-60 drops) per minute, then the transfusion is stopped and the recipient is monitored for 3 minutes, controlling his pulse, respiratory rate, blood pressure, general state, skin color, measure body temperature. This procedure is repeated twice more. The appearance during this period of even one of these clinical symptoms such as chills, back pain, a feeling of heat and tightness in the chest, headache, nausea or vomiting, requires immediate cessation of the transfusion and refusal to transfuse this transfusion medium. The blood sample is sent to a specialized blood service laboratory for an individual selection of red blood cells.
The urgency of transfusion of blood components does not exempt from performing a biological test. During it, it is possible to continue the transfusion of saline solutions.
When transfusing blood and its components under anesthesia, the reaction or incipient complications are judged by an unmotivated increase in bleeding in the surgical wound, a decrease in blood pressure and increased heart rate, discoloration of urine during catheterization Bladder, as well as according to the results of the test for the detection of early hemolysis. In such cases, the transfusion of this blood transfusion medium is stopped, the surgeon and the anesthesiologist-resuscitator, together with the transfusiologist, are obliged to find out the cause of hemodynamic disorders. If nothing but transfusion could cause them, then this hemotransfusion medium is not transfused, the issue of further transfusion therapy is decided by them, depending on clinical and laboratory data.
A biological test, as well as an individual compatibility test, is also mandatory in cases where an individually selected in the laboratory or phenotyped erythrocyte mass or suspension is transfused.
After the end of the transfusion, the donor container with a small amount of the remaining blood transfusion medium used for testing for individual compatibility is subject to mandatory conservation within 48 hours at a temperature of +2 0 С…+8 0 С.
After the transfusion, the recipient observes bed rest for two hours and is observed by the attending physician or the doctor on duty. Every hour his body temperature and blood pressure are measured, fixing these indicators in the patient's medical record. The presence and hourly volume of urination and the color of urine are monitored. The appearance of a red color of urine while maintaining transparency indicates acute hemolysis. The next day after the transfusion, a clinical analysis of blood and urine is mandatory.
In case of outpatient blood transfusion, the recipient after the end of the transfusion should be under the supervision of a doctor for at least three hours. Only in the absence of any reactions, the presence of stable blood pressure and pulse, normal urination, the patient can be released from the hospital.
3.5. Registration of compatibility test results
The result of control tests of samples for compatibility (separately for each type) is entered into the transfusion card of the patient's medical record (see Appendix 2).
3.6. Individual selection
Individual selection of blood gas carriers is carried out in blood service institutions (SPK or OPK) by a doctor of a clinical laboratory diagnostics who have special training in immunohematology on specialized cycles and on the basis of institutions of the blood service of the Republic of Tatarstan.
3.6.1. Indications for individual selection
- aggravated transfusion history (history of post-transfusion reactions or complications to previous transfusions, multiple transfusions)
- aggravated obstetric history (presence in the anamnesis of the birth of children with HDN, stillbirths, miscarriages in the later stages);
- hemolytic disease newborns;
- difficulties in determining the group and / or Rh-affiliation of the patient's blood,
- positive or doubtful result at least one of the tests for individual compatibility or a biological test;
- the presence of isoimmune anti-erythrocyte antibodies of any specificity;
- multiple transfusions.
3.6.2. Requirements for blood samples sent for individual selection
A blood sample for individual selection is taken in two test tubes:
1 test tube - with preservative (3 ml),
2 test tube - without preservative (5 ml).
As a preservative, an EDTA solution or a solution of sodium citrate, 5% is used. The ratio of the test blood and preservative for sodium citrate is 10:1, for EDTA - 100:1.
If individual selection is necessary, two test tubes are allocated to a newborn child:
1 tube - with a blood sample of a child (at least 1.5 ml),
2 tube - with a sample of the mother's blood (5 ml).
Both test tubes must be signed (full name, date of sampling, case history number).
The direction is drawn up in accordance with Appendix No. 3.
Individual selection of blood gas carriers is a preliminary procedure. If the erythrocyte mass or suspension is individually selected for the recipient in a specialized laboratory, the doctor performing the transfusion determines the blood group and Rh affiliation of the recipient, the donor's blood group before transfusion and conducts only one compatibility test on a plane at room temperature and at the beginning of the transfusion - a biological test .
3.7. additional information about the features of performing tests for compatibility
Table 5
| Category of patients | Individual characteristics | The nature of the difficulty | Way to solve the problem |
| newborns | The presence of maternal alloantibodies in the blood of a newborn | At a positive result at least one of the samples, the blood sample of the newborn and the mother is sent to a specialized laboratory for individual selection. | |
| pregnant | The presence of isoimmune anti-erythrocyte antibodies. | Incompatibility in at least one of the samples | A blood sample of the recipient (pregnant, parturient) is sent to a specialized laboratory for individual selection. |
| Patients with hematological or oncological diseases, as well as patients with other types of pathologies (burns, liver cirrhosis, sepsis, etc.) | Presence of non-specific allo- and/or autoantibodies | Incompatibility in at least one of the samples | Carrying out individual tests for compatibility in the gel test or |
| In different categories of patients | The presence of anti-A 1 antibodies (extra agglutinin α 1) | ABO system incompatibility | Sending the recipient's blood sample to a specialized blood service laboratory for individual selection. |
| Massive blood transfusion or transfusion of incompatible blood gas carriers using the ABO system and (or) the Rhesus system | Sending the recipient's blood sample to a specialized blood service laboratory for individual selection. | ||
| Intravenous administration of blood substitute solutions. | Doubtful test results for compatibility | Sending the recipient's blood sample to a specialized blood service laboratory for individual selection. |
Applications
Attachment 1
|
Control date |
Thermometer readings |
Responsible person's signature |
Taken measures |
|
|
Morning |
Evening |
|||
Annex 2
TRANSFUSION CARD No. _______
Date of transfusion ___________________
|
Recipient: BP____________Ps___________Т˚С_____________ Transfusion indicated _________________________ (indicate the name of the transfusion medium) Transfusion history _____________________ _________ Obstetric history _________________________ ___________________________________________ with the aim of ____________________________________ The result of the control check of the blood group according to ABO ____________ Rhesus __________________ |
Donor (auto): (Underline whatever applicable) FULL NAME._____________________________________ Individual No. __________________________ ABO blood group ________ Rhesus ___________ (according to the label) The result of the control check of the donor's blood group according to ABO ______________________________ |
Used diagnostic standards:
1) Standard ABO isohemagglutinating sera or coliclones (underline as appropriate) of the following series:
|
__________/____________Sell by______________ |
__________/____________Sell by______________ __________/____________Sell by______________ |
2) Universal reagent anti-rhesus Rh 0 (D) or anti-D super anti-D super (underline as appropriate) of the following series: ________________ valid until _______________
|
Passport of the transfusion medium |
Compatibility test results |
Transfusion medium transfused |
|||||
|
Manufacturer |
Name |
||||||
|
Ind. blood sampling |
|||||||
|
Preparing for a transfusion |
|||||||
|
Procurement date |
Filtration |
||||||
|
Resuspension |
|||||||
|
Method and rate of transfusion |
|||||||
|
Biol. try |
|||||||
|
Transfusion duration |
|||||||
Observation of the recipient
|
Early post-transfusion period |
First day after transfusion |
||||||
|
During the transfusion |
(for ambulances) |
Urinalysis from _________ |
Blood test from ________ |
Daily diuresis |
|||
|
Allocated |
|||||||
|
Objectively |
|||||||
|
The first portion of urine: |
|||||||
Doctor _______________________________________
Place for gluing Place for gluing results
transfusion medium labels for individual blood collection
Consent of the recipient
for transfusion of blood components
I, ________________________________________________________________________
received an explanation about the blood transfusion operation. The attending physician explained to me the purpose of the transfusion, its necessity, the nature and features of the procedure, its possible consequences, in the event of the development of which I agree to carry out all the necessary medical measures. I have been informed of the probable course of the disease in case of refusal to undergo a transfusion of blood components.
I also received information about alternative methods treatment.
The interview was conducted by doctor _______________ (physician's signature). "___" __________20
The patient agreed with the proposed treatment plan, which he signed with his own hand
______________________________________________ (signature of the patient)
or signed ___________________________________________ (signature, full name)
In the case when the condition of a citizen does not allow him to express his will, and medical intervention is urgent,
The decision of the council of doctors _________________________________
__________________________________
__________________________________
or the signature of the attending (duty) doctor, followed by notification officials medical institution ______________________________________
Instructions for filling out a transfusion card
1. For each transfusion of blood components, including autologous blood, autocomponents, a transfusion card is filled out to the recipient and pasted into the medical record of an inpatient (outpatient) patient in the form of a transfusion card.
2. On the reverse side of the transfusion card, the recipient's consent to the transfusion is recorded in the prescribed form.
3. The number of the transfusion card is indicated in accordance with the ordinal number of the transfusion to the recipient.
4. In the "Recipient" section, the result of the control check before transfusion of the recipient's blood group according to the ABO and Rhesus systems is entered.
5. In the line "Transfusion history" it is necessary to reflect the number of transfusions and reactions to them. In the line "Obstetric history" - the number of pregnancies, childbirth, abortions, the presence of spontaneous miscarriages, stillbirths, hemolytic disease of the newborn.
6. The result of the control re-determination of the donor's blood group according to the ABO system is entered in the "Donor" section. In the case of transfusion of hemostasis and fibrinolysis correctors, as well as immunity correction agents, re-determination of the donor's blood group according to the ABO system is not required.
7. In the section "Passport of the transfusion medium", data on the manufacturer, date of preparation are copied from the label of the transfusion medium. After the end of the transfusion, the label is peeled off from the container with the blood component and pasted onto the back of the transfusion card.
8. In the section "Transfused transfusion medium":
- in the line "Name" the name of the blood component is indicated in accordance with the label;
- in the line "Individual blood selection" indicates the laboratory, institution, whose specialists performed the individual selection;
- in the line "Preparation for transfusion" the method, temperature and duration of preparation of the transfusion medium for transfusion are indicated;
- in the line "Filtration" the type of filter, its manufacturer are indicated;
- the line "Resuspension" will be filled in when 0.9% isotonic sodium chloride solution is added to the container with a blood component before transfusion, indicating its volume, batch number and manufacturer.
- in the line "Volume" the volume of the transfused transfusion medium is indicated.
9. Observation of the recipient is carried out during the transfusion and within two hours after the transfusion. When transfusion is carried out on an outpatient basis, the duration of observation increases to three hours.
Appendix 3
Direction to SPK (OPK)
to determine the group and Rh - accessories, individual selection
( Underline whatever applicable)
Name of medical facility ____________________________ department ___________________
FULL NAME. doctor who sent the blood sample
Contact number______________________________________________________
FULL NAME. patient ________________________________________________________________
Date and year of birth _______________ Medical card number __________________
Diagnosis: _________________________________________________________________
Transfusion history (number of transfusions and reactions to them) __________________
__________________________________________________________________________
__________________________________________________________________________
Obstetric history (number of pregnancies, number of deliveries, HDN of children,
miscarriages, stillbirths, abortions) _______________________________________
_________________________________________________________________________
The results of an immunohematological blood test obtained in a medical facility:
ABO blood group __________________________________
Rhesus - affiliation ________________________________
The presence of anti-erythrocyte alloantibodies ________________
Name of blood components required for transfusion ___________________
____________________________________________________________________________
Date _________________________________ Physician's signature _____________________________
Note: the tube with the blood sample must be marked (full name of the patient, date of blood sampling, medical record number).
If the patient's Hb is below 70 g/l, two test tubes must be taken to determine the group and Rhesus affiliation, for individual selection:
1 tube with preservative (3 ml),
2 tubes without preservative (5 ml).
List of abbreviations
MPU - medical and preventive institution
MH RT - Ministry of Health of the Republic of Tatarstan
Ministry of Health of the Russian Federation - Ministry of Health of the Russian Federation
HDN - hemolytic disease of the newborn
OPK - blood transfusion department
SPK - blood transfusion station
The manual was prepared by Head of the Department of Anesthesiology-Resuscitation and Transfusiology of the State Educational Institution of Higher Professional Education of the KSMA of Roszdrav, Professor V.M. E.A. Sidoruk, assistants of the department R.S. Gadylshina, L.N. Sibgatullina, E.R. Khamidullina, Ph.D. Terekhova, T.V. Ivanova.
Reviewers:
head Department of Surgical Diseases No. 1 with courses oncology, anesthesiology and resuscitation of the State Educational Institution of Higher Professional Education "KSMU Roszdrav", MD, Professor D.M. Krasilnikov;
head Department of Clinical Anatomy and Outpatient Surgery, State Educational Institution DPO "KSMA Roszdrav", Professor V.V. Fattakhov.
Literature:
1. Donskov S.I., Morokov V.A., Dubinin I.V. Group antigens of erythrocytes. Compatibility concept. - M .: "IP Skorokhodov", 2008. - 172 p.
2. Mineeva N.V. Human blood types. Fundamentals of immunohematology. - St. Petersburg: LLC "A-print", 2004. - 188 p.
3. Order of the Ministry of Health of the Russian Federation dated 01/09/1998 No. 2 "On approval of instructions for immunoserology".
4. Order of the Ministry of Health of the Russian Federation of November 25, 2002 No. 363 “On approval of the Instructions for the use of blood components”.
5. Order of the Ministry of Health of the Republic of Tajikistan dated May 13, 1999 No. 367 “On measures to prevent complications during the transfusion of components, blood products, blood substitutes”.
6. Order of the Ministry of Health of the Republic of Tajikistan dated 05.08.2004 No. 1135 “On improving the work on the prevention of post-transfusion complications in healthcare institutions of the Republic of Tatarstan”
7. Order of the Ministry of Health of the Republic of Tajikistan dated April 27, 2004 No. 691 “On the improvement of preconception, prenatal and postnatal prevention of immunoconflict pregnancy in the Republic of Tatarstan”.
8. Order of the Ministry of Health of the Russian Federation dated December 25, 1997 No. 380 “On the state and measures to improve laboratory support for the diagnosis and treatment of patients in healthcare institutions of the Russian Federation”.
9. Order of the Ministry of Health of the Republic of Tajikistan dated July 16, 2004 No. 1047 “On the procedure for obtaining, recording and storing blood components”.
10. Guidelines No. 52001-109 of the Ministry of Health of the Russian Federation, RNII of Hematology and Transfusiology "Requirements for conducting immunohematological studies of donors and recipients at the SEC and in medical facilities", St. Petersburg, 2002
12. Methodological letter of the Ministry of Health and Social Development of Russia dated 10.10.2008 No. 15-4 / 3118-09 “On the procedure for conducting immunohematological studies in pregnant women, women in labor, fetuses and newborns”.
13. Guidelines "Organization of immunoserological studies (determination of blood group and Rh - factor) for patients in the laboratories of medical institutions in Samara and the Samara region."
14. Instructions on the procedure for conducting immunohematological studies of patients in healthcare institutions of St. Petersburg, 2005